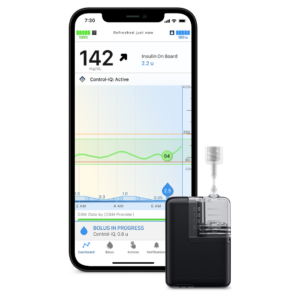
Tandem Mobi System
- Control-IQ + Technology
- AutoBolus Feature
- Mobile App Control (device compatibility)
- Simplified Pairing Process
- User-Friendly Interface
- Dexcom G6 & G7
Specifications
For further warranty information, instructions on appropriate use, and disclaimers, visit the manufacturer website: tandemdiabetes.com
Description
Important Safety Information
RX ONLY.
Indications for Use
Tandem Mobi system: The Tandem Mobi insulin pump with interoperable technology (the pump) is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in persons requiring insulin. The pump is able to reliably and securely communicate with compatible, digitally connected devices, including automated insulin dosing software, to receive, execute, and confirm commands from these devices. The pump is intended for single patient, home use and requires a prescription. The pump is indicated for use in individuals 6 years of age and greater.
Control-IQ+ technology: Control-IQ+ technology is intended for use with compatible integrated continuous glucose monitors (iCGM, sold separately) and alternate controller enabled (ACE) pumps to automatically increase, decrease, and suspend delivery of basal insulin based on iCGM readings and predicted glucose values. It can also deliver correction boluses when the glucose value is predicted to exceed a predefined threshold. Control-IQ+ technology is intended for the management of type 1 diabetes mellitus in persons 2 years of age and greater and of type 2 diabetes mellitus in persons 18 years of age and greater.
Warning: Control-IQ+ technology should not be used in anyone under the age of 2 years old with type 1 diabetes or under the age of 18 years old with type 2 diabetes. It should also not be used in patients who require less than a total daily insulin dose of 5 units of insulin per day or who weigh less than 20 pounds (9 kilograms), as those are the required minimum values needed for Control-IQ+ to operate safely.
Users of the pump and Control-IQ+ must: use the insulin pump, iCGM, and all other system components in accordance with their respective instructions for use. Failure to follow these instructions for use could result in an over delivery or under delivery of insulin. This can cause hypoglycemia (low BG) or hyperglycemia (high BG) events. Visit tandemdiabetes.com/safetyinfo for additional important safety information.
Frequently Asked Questions
Who is responsible for getting the insurance authorizations for medical supplies?
Does the company provide service and supplies to Medicare and Medicaid patients?
Are you a participating provider with other insurance companies?
Are all products covered by my insurance company?
How much will a patient have to pay for his or her product?
Do I need to apply for Medicare when I turn 65?
Who can receive benefits for diabetes self-management training?
How do I know my diabetes supplies are covered by Medicare?
How much is reimbursed for each product?
Does everyone pay the same amount for the Part B premium?
Are there limits on the quantity of diabetes supplies that Medicare will reimburse?
How do I replace my Medicare card?
If I am 65 and ready to retire and my employer is going to provide me with benefits, do I need Medicare?
Does Medicare cover my spouse and family?
What is the difference between Medicare and Medicaid?
Medicaid is an assistance program. It serves low-income people of various ages. Medical bills are paid from federal, state and local tax funds. Patients usually pay no part of the costs or very little for covered medical expenses, although a small co-payment is sometimes required. Medicaid is a federal-state program. It is run by state and local governments within federal guidelines, so it varies from state to state.
Medicaid es un programa de asistencia. Atiende a personas de bajos ingresos de distintas edades. Las facturas médicas se pagan con fondos procedentes de los impuestos federales, estatales y locales. Los pacientes suelen pagar poco o nada por los gastos médicos cubiertos, aunque a veces se exige un pequeño copago. Medicaid es un programa federal-estatal. Lo gestionan los gobiernos estatales y locales dentro de las directrices federales, por lo que varía de un estado a otro.